Contents
- Introduction
- Who has to register?
- General exceptions and exemptions from registration
- The regulated activities
- Glossary of terms
The regulated activities
The regulated activities are detailed in Schedule 1 of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014.
We describe each regulated activity and give some examples of services that are likely to carry on these activities. If the general exceptions and exemptions do not apply to you, you must register for each regulated activity that you provide, unless a specific exemption applies.
You need to be sure that the service you provide is covered by the regulated activities you register for. To do this, check all the activities, read the guidance and use the diagrams to help you decide if you need to register for that activity.
The Quick reference guide also shows which regulated activities you are likely to need to register for.
You may need to register for more than one regulated activity to cover the service(s) you provide. Some providers may need to register for several regulated activities.
How the regulated activities relate to each other
Each regulated activity requires a separate registration.
There is no hierarchy of regulated activities — they are all equally important and you must apply for all that relate to your service.
Sometimes, registration for one regulated activity will remove the need to register for another. For example, a provider will not need to apply for:
- Nursing care where it is part of another regulated activity (such as Treatment of disease, disorder or injury)
- Personal care where it is delivered as part of:
- Accommodation for persons who require nursing or personal care
- Accommodation for persons who require treatment for substance misuse
- Treatment of disease, disorder or injury.
However, wherever nursing care or personal care is provided in its own right (not as part of another regulated activity), then a provider may need to register for it as a regulated activity, even if the provider is registered for other regulated activities.
This section contains:
- Personal care
- Accommodation for persons who require nursing or personal care
- Accommodation for persons who require treatment for substance misuse
- Treatment of disease, disorder or injury
- Assessment or medical treatment for people detained under the Mental Health Act 1983
- Surgical procedures
- Diagnostic and screening procedures
- Management of supply of blood and blood-derived products
- Transport services, triage and medical advice provided remotely
- Maternity and midwifery services
- Termination of pregnancies
- Services in slimming clinics
- Nursing care
- Family planning services
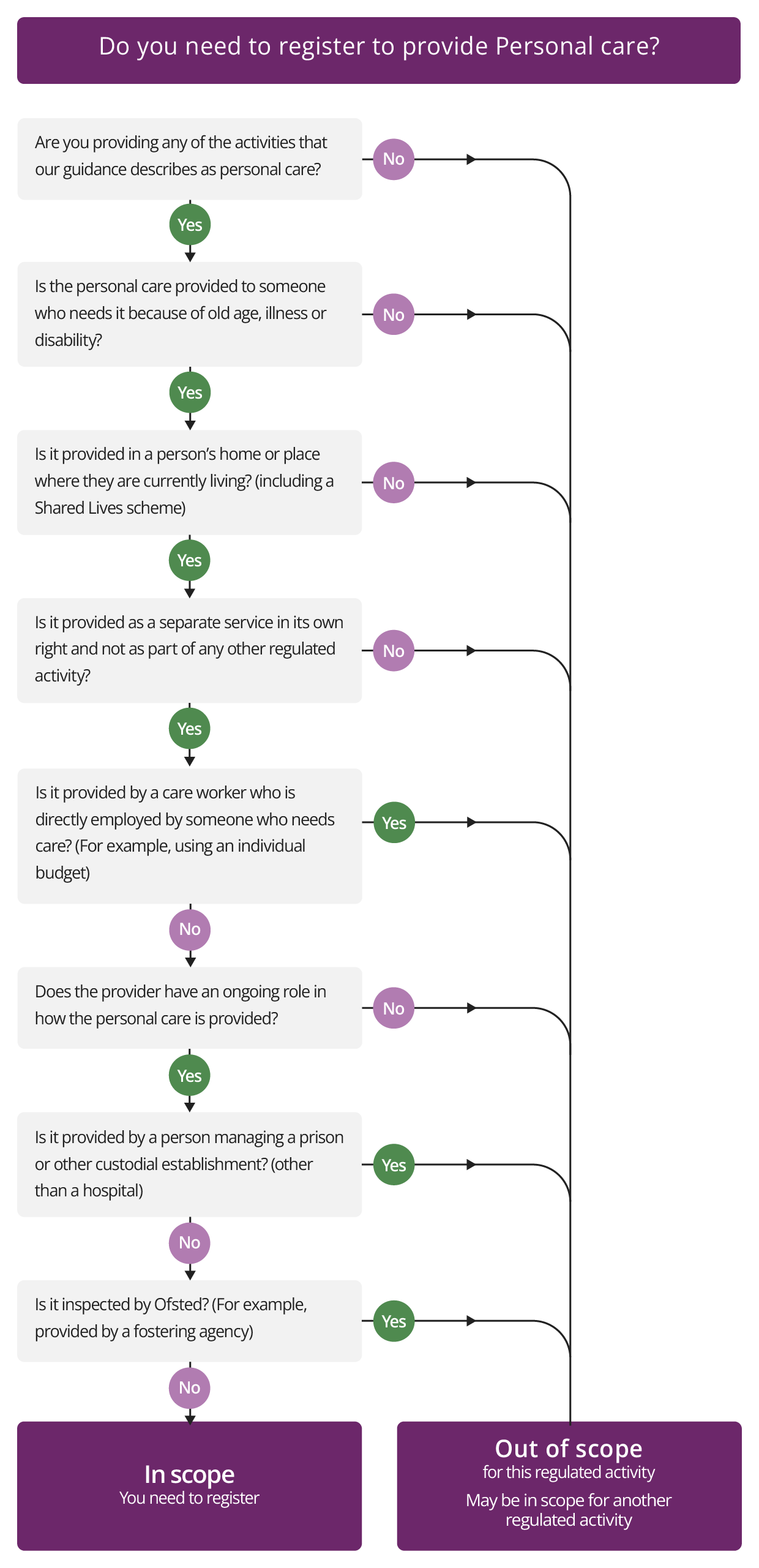
Personal care
Description
Personal care is defined in Regulation 2 (Interpretation) of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014. See the definition of personal care in our glossary of terms.
The regulated activity of Personal care involves providing personal care for people who are unable to provide it for themselves because of old age, illness or disability. The personal care must be provided in the place where those people who need it are living at the time when the care is provided.
For example, this includes personal care provided through domiciliary or homecare services, and housing with care or supported living services. Sometimes, people receiving personal care live in accommodation where it is a requirement of occupation that they both need and receive a care service while living there. For the care service to be correctly registered for the regulated activity of Personal care, there must be a real separation between the provision of personal care and the accommodation agreements. See additional guidance on supported living and extra care housing services.
This regulated activity also includes Shared Lives schemes (see what this means in our glossary of terms) where the provider of the scheme is registered for personal care – not the owners of the individual homes (the accommodation). If you are carrying on the regulated activity of Treatment of disease, disorder or injury, you do not also need to register for Personal care if you deliver this as part of the treatment. However, if you provide personal care to people who are not also receiving treatment for a disease, disorder or injury, you will need to register for Personal care.
When this regulated activity does NOT apply
This regulated activity does not apply if your service does not provide the activities defined as personal care. See Regulation 2 and Schedule 1(1) of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014 for full details.
It is the nature of the care (activity) being provided that determines the need for registration and which regulated activity is applicable.
For example, you do not need to register if you only provide housing support or social support (such as help with shopping), but no tasks that are included in the definition of personal care. Similarly, you do not need to register for the activity if you only provide support to administer, prompt or supervise medicines but do not intend to provide any of the activities defined as personal care.
Examples of some specific exceptions
You are excepted from this regulated activity and do not need to register for Personal care if:
- You are a provider managing a prison or other similar custodial establishment (apart from a hospital within the meaning of Part 2 of the Mental Health Act 1983) and you provide personal care for people who are detained. A prison is considered to be where a person is living for the time that they are detained there. This means that if a domiciliary or homecare agency provides personal care in prisons or similar custodial settings, the provider of the domiciliary or homecare agency must register with us because the exemption only applies to the person managing the prison.
- You are a fostering agency that is inspected by Ofsted, and your services include providing personal care to children who are placed or being placed with foster carers.
- You are registered or registering to provide accommodation together with personal care for the people who use your service in a care home setting (the regulated activity of Accommodation for persons who require nursing or personal care). However, if you also intend to provide personal care services to people in places where they live (for example, in their own homes) as well as providing accommodation together with personal care, then you will also need to apply separately for the regulated activity of Personal care.
- Your role is an employment or introductory agency, and you supply care workers:
- to another organisation who will then be responsible for directly providing the care, or
- to a person who will then take whole responsibility for arranging to provide their own care under a personal budget or private arrangement.
See further information about people who introduce a care worker to an individual but then have no ongoing role in the personal care that a carer provides after they are introduced.
- You are a carer or personal assistant directly employed by a person or a related third party (without the involvement of an employment agency or employment business) and you work wholly under the direction and control of that person or related third party to meet the person’s own personal care requirements. See the definition of a related third party in our glossary of terms.
Shared lives schemes (previously known as adult placement schemes)
Shared lives schemes (referred to in the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014) should register only for the regulated activity of Personal care and not the regulated activity Accommodation for persons who require nursing or personal care. This is because:
- the provider of the scheme is registered and not the owners or providers of the individual homes (the accommodation)
- the accommodation aspect of the service supplied by the shared lives carer is out of the scope of the regulations, and the homes where people live are not 'regulated premises' that we can inspect.
Shared lives schemes should only register for Personal care where they provide placements for people with personal care needs. If they do not provide this type of placement, they will be out of scope for this regulated activity. See what we mean by shared lives schemes in our glossary of terms.
Additional guidance for Personal care
Our Housing with care guidance gives more information about regulated activities for personal care services provided to people living in specialist schemes such as supported living and extra care housing.
Personal care: ongoing role, introductory agencies and individual care workers
Check if you need to register for Personal care
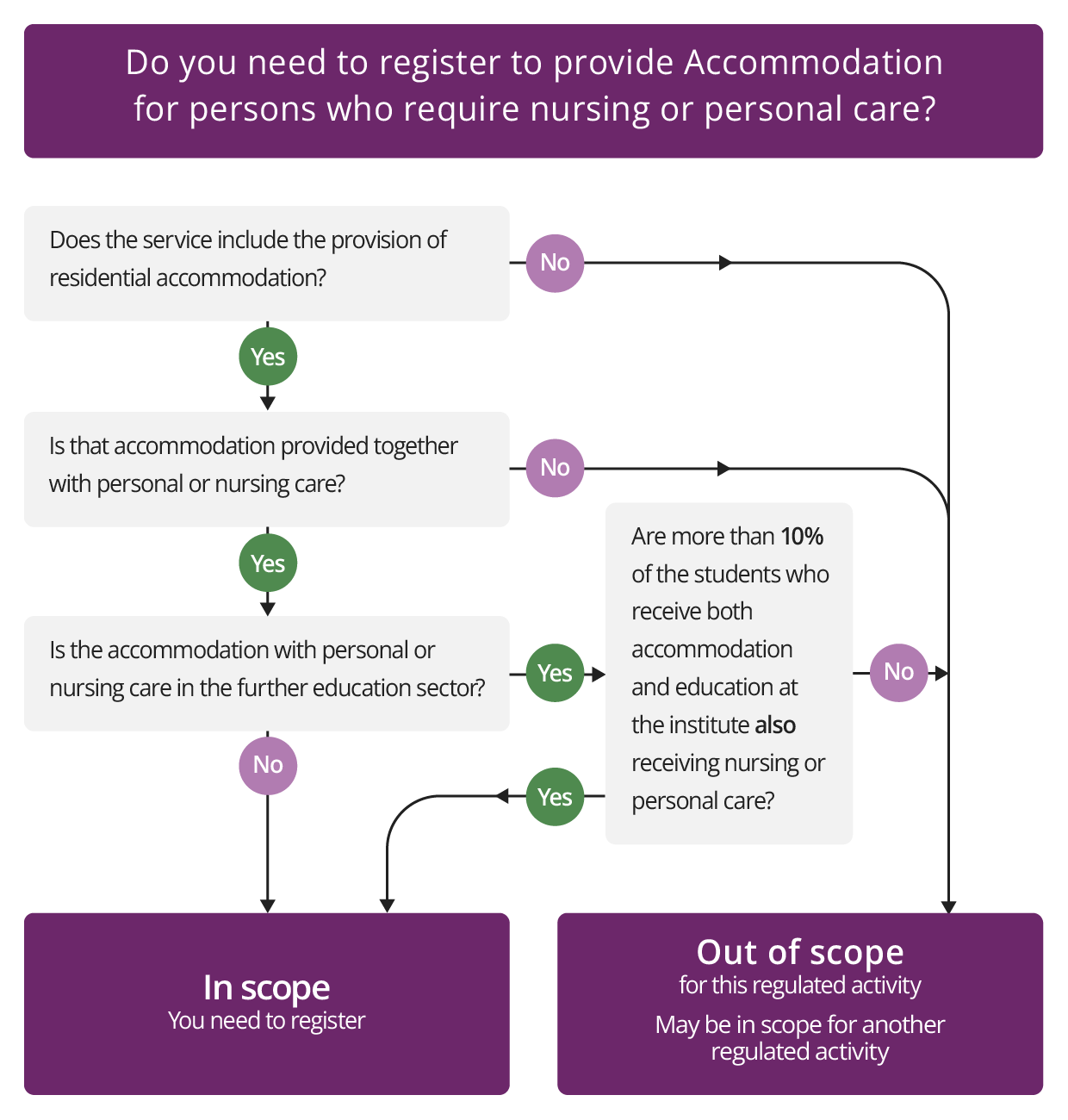
Accommodation for persons who require nursing or personal care
Description
This regulated activity applies where residential accommodation is provided together with nursing care or personal care as a single package, for example nursing or personal care delivered in a care home setting.
A single package means the person using the service cannot choose to receive personal care from another provider while they are living in the accommodation. In the same way, to receive the accommodation they are required to receive their personal care from one specified provider. The accommodation and the care will usually be from the same provider, but they do not need to be, as long as any contractual arrangements make clear who is responsible for carrying on this regulated activity.
If accommodation and personal care are provided separately and people living in the accommodation can choose a different provider to meet their personal care needs, then it may be a supported living or extra care housing service. In this case the regulated activity of Personal care may apply.
You do not have to additionally register for the activities of Personal care or Nursing care if you provide the regulated activity of Accommodation for persons who require nursing or personal care. Where someone living in a care home needs nursing care, this may be provided by care staff where the tasks can be delegated appropriately by a listed healthcare professional (see what this means in our glossary of terms) who is employed by a provider registered to carry on the regulated activity of Treatment of disease, disorder or injury (for example, a district nursing service). See more information on delegation in Treatment of disease, disorder or injury.
You may need to apply for other regulated activities where these apply. For example, providers of care homes with nursing are likely to need to also register for Treatment of disease, disorder or injury if they employ registered nurses or other listed professionals who carry on this regulated activity. There may be exceptions to this principle, but only when registered nursing staff are not employed in their professional capacity and do not actually carry out the treatment for a disease, disorder or injury.
Further education sector
In some cases, this activity includes accommodation together with personal or nursing care provided in an establishment in the further education sector. For this activity to apply in the further education sector, more than 10% of the students receiving both accommodation and education at the establishment must also be receiving personal or nursing care. We will normally judge this by looking at the number of students over a 12 month period, rather than just on a single day.
An establishment in the further education sector means an establishment conducted by a further education corporation, or an establishment designated as such by an order of the Secretary of State for Education.
This activity does not include providing accommodation for people who require nursing or personal care in schools.
Shared lives
If you provide a shared lives service, you should register only for the regulated activity of Personal care and not for the regulated activity of Accommodation for persons who require nursing or personal care. See more information on shared lives schemes under the Personal care activity.
Check if you need to register for Accommodation for persons who require nursing or personal care
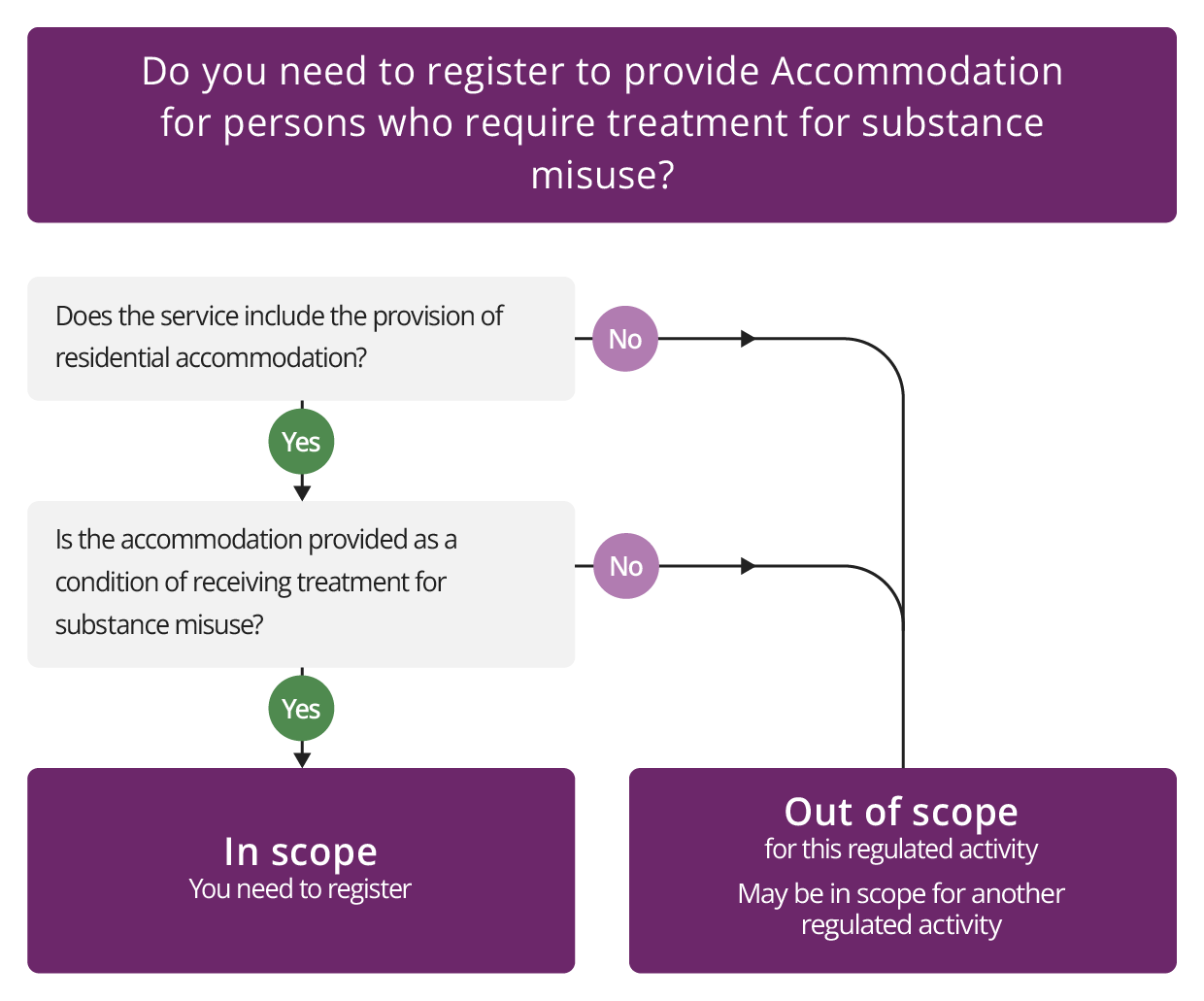
Accommodation for persons who require treatment for substance misuse
Description
This regulated activity consists of residential accommodation for people together with treatment for substance misuse.
Treatment
In this regulated activity, ‘treatment’ covers a range of recognised treatment interventions, such as managed withdrawal or detoxification, or a structured psychosocial treatment programme. It is not limited to treatment provided by a healthcare professional. These types of treatment will always trigger the need to register for this regulated activity if they are provided together with residential accommodation.
Accommodation
The activity covers residential accommodation – this is not the same as hospital accommodation where people receive detoxification treatment. For this activity to apply, a service provider must provide the accommodation 'together with' treatment to the same residents. This means that this activity does not apply to hospitals that provide detoxification treatments for substance misuse. The detoxification being provided in the hospital would be covered under the activity of Treatment of disease, disorder or injury.
The treatment for substance misuse does not necessarily need to be provided in the same place as the accommodation, it could be on a different site. For example, the treatment may be delivered in a community setting such as a day centre or community centre, with the people accommodated in separate facilities somewhere else. However, the accommodation and the treatment must be linked so that the accommodation is provided because someone requires and accepts treatment.
Related regulated activities
Personal care and Nursing care:
You do not have to additionally apply to register for the regulated activities of Personal care or Nursing care if you provide this activity. This is because they would be covered as part of the treatment you provide for the substance misuse. The only exception to this would be if you also provide personal or nursing care as a separate service (for example a domiciliary or homecare service).
Treatment of disease, disorder or injury:
In the same way, you do not also have to apply to register for Treatment of disease, disorder or injury. This is because the treatment for substance misuse is covered under the activity of Accommodation for persons who require treatment for substance misuse. You would only have to apply for Treatment of disease, disorder or injury if you provide other treatments that are separate from the treatment of substance misuse. For example, treating substance misuse includes detoxification, but you would also have to register for Treatment of disease, disorder or injury if:
- a doctor from the team treats a medical condition unrelated to the substance misuse, or is treating an eating disorder
- a registered nurse was managing a holistic care plan for a dual diagnosis patient and administering treatment for both mental illness and for substance misuse.
Check if you need to register for Accommodation for persons who require treatment for substance misuse
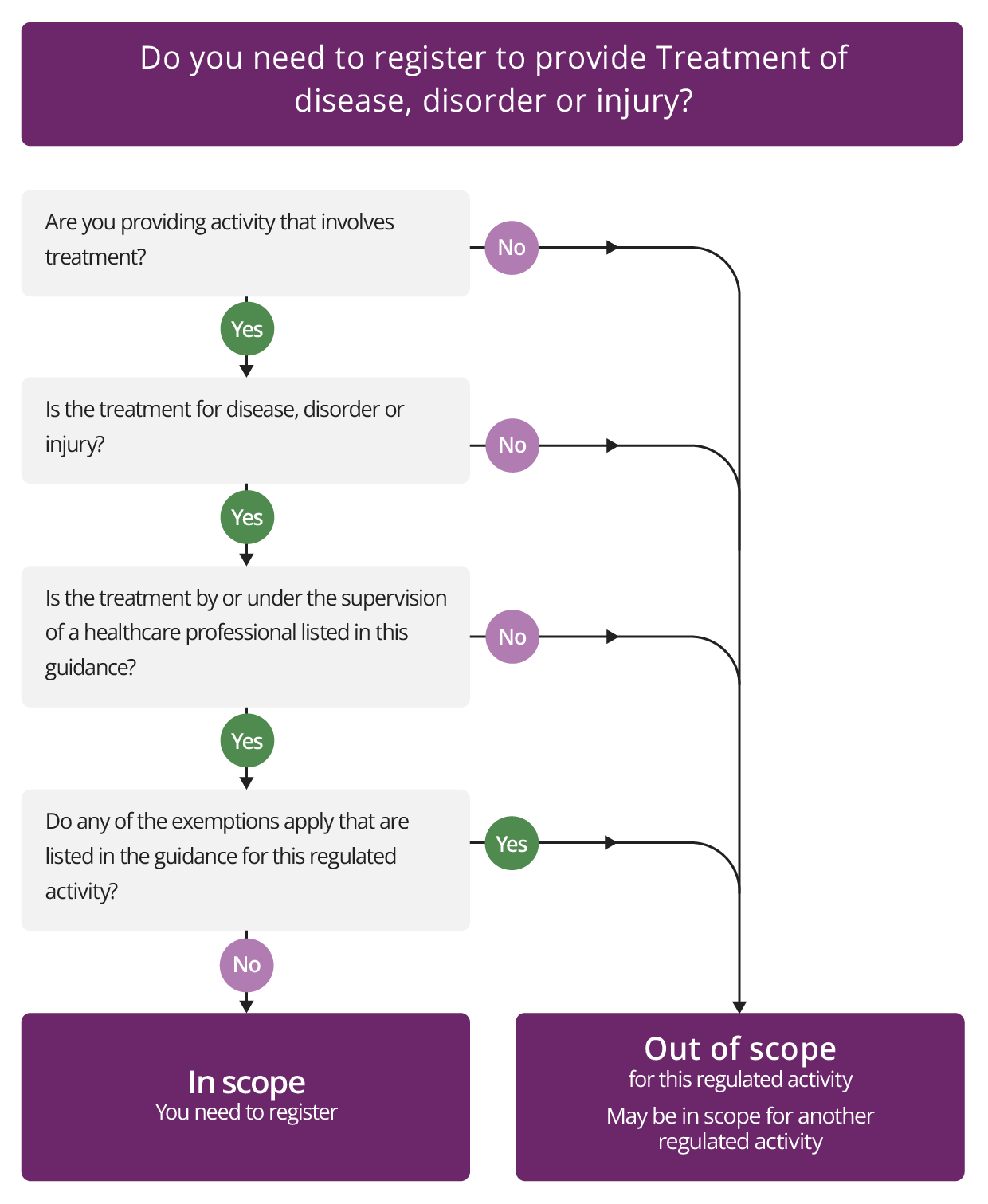
Treatment of disease, disorder or injury
Description
This activity covers a treatment that is:
- provided by or under the supervision of a defined list of healthcare professionals (see what this means in our glossary of terms) or by a multi-disciplinary team that includes a listed healthcare professional, or
- provided by or under the supervision of a social worker where the treatment is for a mental disorder, or by a multi-disciplinary team that includes a social worker where the treatment is for a mental disorder, and is
- for a disease, disorder or injury.
Treatment of a disease, disorder or injury covers a wide range of treatments. We don’t provide a complete list here, but it includes examples such as:
- emergency treatment
- ongoing treatment for long-term conditions
- treatment for a physical or mental health condition or learning disability
- giving vaccinations or immunisations
- palliative care.
This regulated activity applies to the treatment of disease, disorder or injury in any setting, for example hospitals, clinics, hospices, ambulances, GP and dental surgeries, community services, and care homes.
What this regulated activity does NOT include:
- Interventions carried out purely for cosmetic purposes.
- Alternative and complementary medicine, with the exception of the practice of osteopathy or chiropractic.
- First aid where it is delivered by:
- healthcare professionals in unexpected or potentially dangerous situations requiring immediate action
- non-healthcare professionals who are trained to deliver first aid
- organisations established for the purpose of providing first aid
- Treatment provided in a sports ground or gymnasium (including associated premises) where it is provided for the sole benefit of people taking part in, or attending, sporting activities and events.
- Treatment provided through temporary arrangements for sporting or cultural events (such as festivals, sporting or motor sport events).
- Hyperbaric oxygen therapy provided to workers in connection with their work or when governed by the Diving at Work Regulations 1997 or Work in Compressed Air Regulations 1996.
- Activities authorised by a licence granted by the Human Fertilisation and Embryology Authority.
Read Schedule 1 (4)(3) of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014 for the full list of excluded activities.
Sometimes, Treatment of disease, disorder or injury is provided as only one part of a service, for example in a large care home that has just a few intermediate care or specialist palliative care beds. If the activity is carried out by or under supervision of a listed healthcare professional employed by the provider, you will still need to register for it in addition to any other activities that you may need to register for your service.
If another body provides those services, our guidance on hosted services in Who has to register? applies.
Other regulated activities covered by this regulated activity
If you are registered for Treatment of disease, disorder or injury, and when delivering it you provide:
- Personal care
- Nursing care
you do not need to apply for those additional regulated activities.
Additional regulated activities you may need to register for
You should also apply for other regulated activities if you are providing them. Examples might include, but are not limited to:
- Surgical procedures
- Diagnostic and screening procedures (where not provided as part of a treatment)
- Assessment or medical treatment for persons detained under the Mental Health Act 1983
- Services in slimming clinics.
Who must carry on the regulated activity for it to be in scope
You will need to register for this regulated activity if your service includes treatment that is carried out by:
- a listed healthcare professional (see what this means in our glossary of terms), or if this professional supervises the treatment, or
- a social worker where the treatment is for a mental disorder, and is intended to treat disease, disorder or injury.
See the specific definition of a healthcare professional for this regulated activity only.
If a multi-disciplinary team includes one of these healthcare professionals (or a social worker in the case of mental health treatment) involved in their professional capacity, then the activity will be within scope and needs to be registered.
Where the situation is unclear, we will consider each case individually. For example:
- If the person providing treatment is not acting in the capacity of a listed healthcare professional, even if they hold a professional qualification. For example, a beautician who is also a registered nurse may be carrying out a cosmetic or aesthetic service. Similarly, a psychiatrist may also be qualified as a psychotherapist. Where they practise as a psychiatrist (a registered medical practitioner) it may be in the scope of this activity. Where they practise solely as a psychotherapist, it would not be in scope of this activity.
- Where it is not clear whether the service is treatment or is being carried out for another reason. For example, some interventions that are normally aesthetic, such as laser hair removal, may also be carried out in response to a clinical disorder.
- In community mental health care (including primary mental health care) some psychological therapies may be provided by healthcare professionals, social workers or by others with specific qualifications. In these cases, some service providers will need to register, whereas others will not. This depends on whether they use healthcare or social work professionals to deliver or supervise treatment.
Professionals who are NOT included in the scope of this regulated activity
The list of professionals in the regulations does not include:
- clinical psychologists
- occupational therapists
- physiotherapists
- pharmacists
- opticians
- dietitians
- nursing associates.
If you are one of these professionals and you run a standalone treatment service, you do not need to register for Treatment of disease, disorder or injury.
Social workers
Most (but not necessarily all) specialist mental health services provided by social workers who are working in their professional capacity as a social worker will be within the scope of this regulated activity, and the provider of that service will need to register. For example, where treatment includes ongoing assessment of a person’s mental state or where the social worker is providing a psychological therapy. Other types of social work services will not be in the scope of this regulated activity.
Nursing associates
The role of nursing associate introduced in 2019 is not included in the list of healthcare professionals who can carry out activities covered by the regulated activity of Treatment of disease, disorder or injury. This means that a provider cannot register for this regulated activity based on the employment of nursing associates alone, though they may be employed to work as part of a nursing team with other listed healthcare professionals.
What we mean by treatment "under the supervision of" a listed healthcare professional or social worker
A person’s treatment is ‘under the supervision of’ a healthcare professional (as listed in paragraph 4(4) of Schedule 1 of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014) where the healthcare professional:
- is part of the team that directly provides health care to the person, or
- directly reviews the person's case and sets out a plan of care for them even if this plan is carried out by another person, or
- authorises a protocol of care, which is used by other care givers, that:
- they are directly accountable for, and
- they are required to continually monitor, and
- can only be authorised by the healthcare professional by virtue of their professional registration, and
- directly records the details of the people who receive care when the protocol is used.
Where a service solely produces Patient Group Directions (PGDs) (see what this means in our glossary of terms), it is not carrying on a regulated activity. This is because there is no treatment ‘by or under the supervision of’ a healthcare professional from the service that produces the PGD, even though a healthcare professional may be involved in creating and/or authorising the PGD itself.
‘Delegation’ of healthcare tasks
Delegation is different to supervision. A healthcare professional employed by a provider registered for Treatment of disease, disorder or injury can delegate a procedure to a care worker or nursing associate who is employed by a second provider operating, for example, a care home without nursing or a domiciliary or homecare care agency. The healthcare professional, for example a nurse employed by a community trust, must ensure that a task is appropriately delegated and assess that it is within the worker’s competence.
The second provider cannot be considered to be carrying on this regulated activity and is not required to register for it, as they are not employing healthcare professionals to provide or supervise the activity. The first provider remains responsible for the treatment of a person using the second provider’s service, including the outcome of any healthcare task they delegate to staff working for the second provider. However, a provider cannot be responsible for the day-to-day supervision of staff who are employed by another provider. It is therefore the second provider’s responsibility to ensure that they do not allow their staff to accept delegated tasks unless the staff have enough support, supervision, education and training to competently undertake the aspects of care that are being delegated.
The professional standards that registered nurses, midwives and nursing associates must uphold are set out in The Code: Professional standards of practice and behaviour for nurses, midwives and nursing associates 2019 (Nursing and Midwifery Council). Registered nurses, midwives and nursing associates must act in line with the Code. This includes requirements about accountability for decisions to delegate tasks and duties, and for the outcome of the delegated tasks. The Royal College of Nursing also provides guidance on accountability and delegation.
Administering medicines
The activity of Treatment of disease, disorder or injury will apply:
- In a situation when a listed healthcare professional (see what this means in our glossary of terms) is required to either prescribe or administer medicines.
- When the listed healthcare professional (see what this means in our glossary of terms) is required to have knowledge or training to administer the medicines, or when a person would not normally be considered able to administer the medicine by themselves.
- When administering any intravenous (IV) product that includes any one or all of: mathematical calculation, reconstitution and use of equipment for the purpose and subsequent care of the patient and IV site.
- When administering chemotherapy in a person’s home, a residential home, or in any other setting. This will require training in administration, IV pumps, checking blood results, examination and care of the IV site, and what to do if there is extravasation (unintentional leaking of vesicant medicines from the vein into the surrounding tissue causing blistering and tissue injury).
The activity will not apply:
- When a care worker or a healthcare professional administers medicines that have either been prescribed and dispensed for a person, or are a 'homely remedy' (including over-the-counter medicines, complementary therapies and herbal preparations), where:
- the person using the service would normally be able to administer the medicines for themselves, including drawing up and injecting insulin and taking a controlled drug orally, but because of circumstances or personal choice, they have consented for the medicine to be administered to them, or
- the person would normally be able to administer the medicines for themselves but because of circumstances, or where they are unable to make a decision about taking their medicines, it has been agreed to be in their best interest for the medicine to be administered to them.
- When a care worker prompts and/or supervises a person to take their prescribed medication.
- When a care worker or nursing associate cares for people receiving nutritional support through a PEG feed or provides care for pressure areas delegated by a district registered nurse (a listed healthcare professional) who works for another provider.
Professional roles and protected titles
The use of professional titles is protected by law. If a person uses a professional title, the assumption will be that the service is being carried out by someone acting in their capacity as a registered healthcare professional and directly using their professional qualification. This means that if the profession is one of those on the list in the regulations, registration will usually be required.
If we find that the service does not involve the professional qualification, and the service may be using the protected title inappropriately in the description of the service or solely for other purposes (such as marketing), we may refer the matter to the relevant professional regulator.
You must consider whether the person providing the service is using their professional qualification in the job they are employed to do. For example, where a care home without nursing has employed a registered nurse or other healthcare professional as a care worker. Where the employee’s qualification is coincidental or only relevant as background knowledge, it will not trigger the need for registration.
However, in this case the health professional should not be using their professional registration status or presenting themselves as that health professional.
Examples: A qualified psychoanalyst, who is also qualified as a psychiatrist but is not prescribing or using medical interventions, will not need to register for their psychoanalytic practice. They are acting in their capacity as a psychoanalyst (not in the list of healthcare professionals) and not as a doctor. They are not using the specific skills taught in medical training but are using the specific skills taught in psychoanalytic training. They are not using statutory authority or powers, which require a medical qualification (for example, prescribing). They are not presenting themselves as a psychiatrist, but as a psychoanalyst.
A drug and alcohol worker, who is also qualified as a social worker, will not need to register to provide a service where they are only working as a drugs worker. However, if they are working as a social worker then the service will need to register. We can tell whether they are working as a social worker if they are clearly using the specific skills taught as part of social work training, or they are presenting themselves as a social worker and using that protected title to describe their work.
Registering for specific services
Lasers and intense pulsed light (IPL)
Healthcare professionals and beauty therapists often use lasers and IPLs for non-surgical procedures. For example, in cosmetic procedures such as hair removal, and for therapeutic procedures such as minor dermatological conditions.
These procedures can constitute appropriate treatment of recognised medical disorders and beauty therapists who are trained to use the laser or IPL can carry out the procedures safely and appropriately. Dermatologists sometimes refer or advise patients to visit a beauty therapist. This is because, even in a clinical service such as dermatology or plastic surgery, very few laser or IPL procedures require the skills of a healthcare professional.
Use of lasers and IPLs is not part of professional training in healthcare professions. Although a healthcare professional’s knowledge of physiology and physical and mental conditions may add value to a service, it is usually useful as background knowledge rather than using their professional training.
If you provide laser and IPL services that are delivered by listed healthcare professionals, you will only need to register where:
- the specific skills of a listed healthcare professional are used, for example where the service is part of a package of clinical care and requires specialist physiological and psychological knowledge such as use of a laser as part of plastic surgery procedures (in this case the regulated activity of Surgical procedures would apply), or
- the service is combined with other procedures that require a listed healthcare professional qualification, for example prescribing, or
- you describe the service as being carried out by someone acting in their capacity as a registered healthcare professional.
Intravenous (IV) products
Intravenous administration of vitamins and products that are prescription only medicine (including 0.9% saline) that are used to improve or enhance wellbeing constitutes treatment of a disease or disorder. We consider a ‘disease’ to include a pathophysiological response to internal or external factors, and a disorder to include a disruption to regular bodily structure and function.
If you provide intravenous administration services you will need to register for the regulated activity if the procedures you offer:
- are delivered by, or under the supervision of, a listed healthcare professional and
- include administering prescription-only products intravenously or products that require a prescription when delivered in intravenous form and
- claim to alter a person’s physiological state in response to a defined concern.
We do not consider this type of procedure to be alternative or complementary medicine.
Orthodontics – clear aligner treatment
Clear aligner treatment is an orthodontic treatment that corrects misaligned or crooked teeth using clear dental appliances. We consider the treatment planning and diagnosis associated with aligners to be a regulated activity, regardless of how the treatment is initiated. If you are a provider of clear aligner treatment, you are likely to need to register for the regulated activity of Treatment of disease, disorder or injury.
Treatment of obesity
The regulated activity of Services in slimming clinics only applies where a medical practitioner provides or supervises advice or treatment in a clinic, including prescribing medicines, for the purposes of weight reduction.
Treatment of disease, disorder or injury would apply where a medical practitioner provides treatment of obesity other than in a clinic (for example, through online services).
If you are another type of listed healthcare professional (see what this means in our glossary of terms) and you treat people for obesity, this is included in the regulated activity of Treatment of disease, disorder or injury. We interpret the term ‘treatment of obesity’ to include:
- using a medicine prescribed for this purpose
- supervising people’s treatment for obesity with a medicine prescribed for this purpose
- treating people in a clinic or through an online web-based service.
Contact us if you are not sure whether the obesity treatment you deliver needs to be registered, or if you are registered for the correct regulated activities.
Earwax removal treatments
Earwax build-up is a natural physiological problem that can result in a health problem or can worsen the symptoms of an existing medical condition, leading a person to seek assistance from a professional. Whichever type of treatment is used, earwax removal is a regulated activity if:
- the person and a listed healthcare professional both agree that there is a problem that needs an intervention; and
- the treatment is carried out by a listed healthcare professional.
You will need to register for Treatment of disease, disorder or injury if this applies.
First aid
You do not need to register if you only provide first aid as this is not regulated activity.
First aid is:
- the initial response to a sudden illness, condition or injury or exacerbation of an existing illness
- restricted to the aim of either alleviating it immediately through simple procedures and/or preventing it from worsening until professional medical help is available.
First aid may include simple non-invasive physiological monitoring techniques carried out as part of the overall first aid care and be provided by lay people or healthcare professionals.
If a healthcare professional administers first aid, they will not need to rely on their specific area of professional expertise but will only use skills and knowledge that will reasonably be expected of any person who has received training in first aid.
A first aid service may involve:
- Healthcare professionals, but only where they are acting in their capacity as a first aider alone. For example, they are not prescribing, following a patient group directive, using specialist drug administration techniques, or using other specialist skills that reflect their professional training rather than their first aid training.
- Simple procedures for assessment that:
- do not need to use a recognised professional diagnostic qualification (for example, as a radiographer or sonographer) and
- are limited to only assessing the need for onward referral for treatment. This includes use of an electrocardiogram (ECG), automated non-invasive blood pressure measurement, pulse oximeter, use of a thermometer, sphygmomanometer or ophthalmoscope.
- Urgent procedures that can be carried out by volunteers. For example, where volunteers, including community first responders, are trained to carry out cardiopulmonary resuscitation (CPR) and use an automatic external defibrillator (AED) (sometimes called a community defibrillator or public access defibrillator (PAD)).
Care homes with nursing
If you are a provider of a care home with nursing, you are likely to need to register for this regulated activity. This is because you will probably employ registered nurses or other listed healthcare professionals who carry out these activities. There may be exceptions to this rule, but only when the registered nursing staff do not actually carry out or supervise the treatment for a disease, disorder or injury.
Care homes without nursing
Providers of care homes without nursing should not register for this regulated activity. This is because if you are carrying out these treatments in the care home, it will likely be by delegation from a healthcare professional working for another registered provider. This includes where you employ registered nursing associates, but they are carrying out treatment delegated by a listed healthcare professional such as a district registered nurse who is employed by another provider.
Treatment for substance misuse
The types of listed healthcare professionals that most commonly work in substance misuse services are:
- medical practitioners
- registered nurses
- social workers.
If any of these professionals are working in their registered capacity (under their protected professional titles) and providing treatment as part of a multi-disciplinary team, it means the whole team will be within the scope of registration as providers of this regulated activity. If you employ them you should register as your service is providing treatment for a disease, disorder or injury.
If your service provides substance misuse treatment but the team does not include a relevant listed healthcare professional working in their registered capacity, then you do not need to be registered for this regulated activity. For example, if you employ staff as drug and alcohol workers as part of a community based multi-disciplinary drug and alcohol team, but the team does not include a healthcare professional such as a medical practitioner, registered nurse or social worker, then you do not need to register for this activity.
If your service does not provide residential accommodation but people are receiving detoxification treatment delivered by a listed healthcare professional working in their professional capacity, then you should be registered for the regulated activity of Treatment of disease, disorder or injury.
School nursing and health visiting
If you provide school nursing and health visiting services, you are likely to need to register for this regulated activity as you employ nurses or other listed healthcare professionals who deliver these activities. This can be the case even where the services delivered do not initially appear to include any treatment for a disease, disorder, or injury (for example, a baby weighing clinic). This is because treatment includes the ongoing assessment of a person’s physical or mental state and giving vaccinations or immunisations. This does not apply where a nurse is directly engaged and directed by a school.
Research settings
Treatment of disease, disorder or injury may also be provided in research settings, sometimes as a secondary purpose. You should register for this regulated activity where the research forms part of a person’s treatment for a disease, disorder or injury, and is carried out by or under supervision of a listed healthcare professional. Research organisations that carry out clinical trials on people who are not being treated will not be required to register for this activity.
Check if you need to register for Treatment of disease, disorder or injury
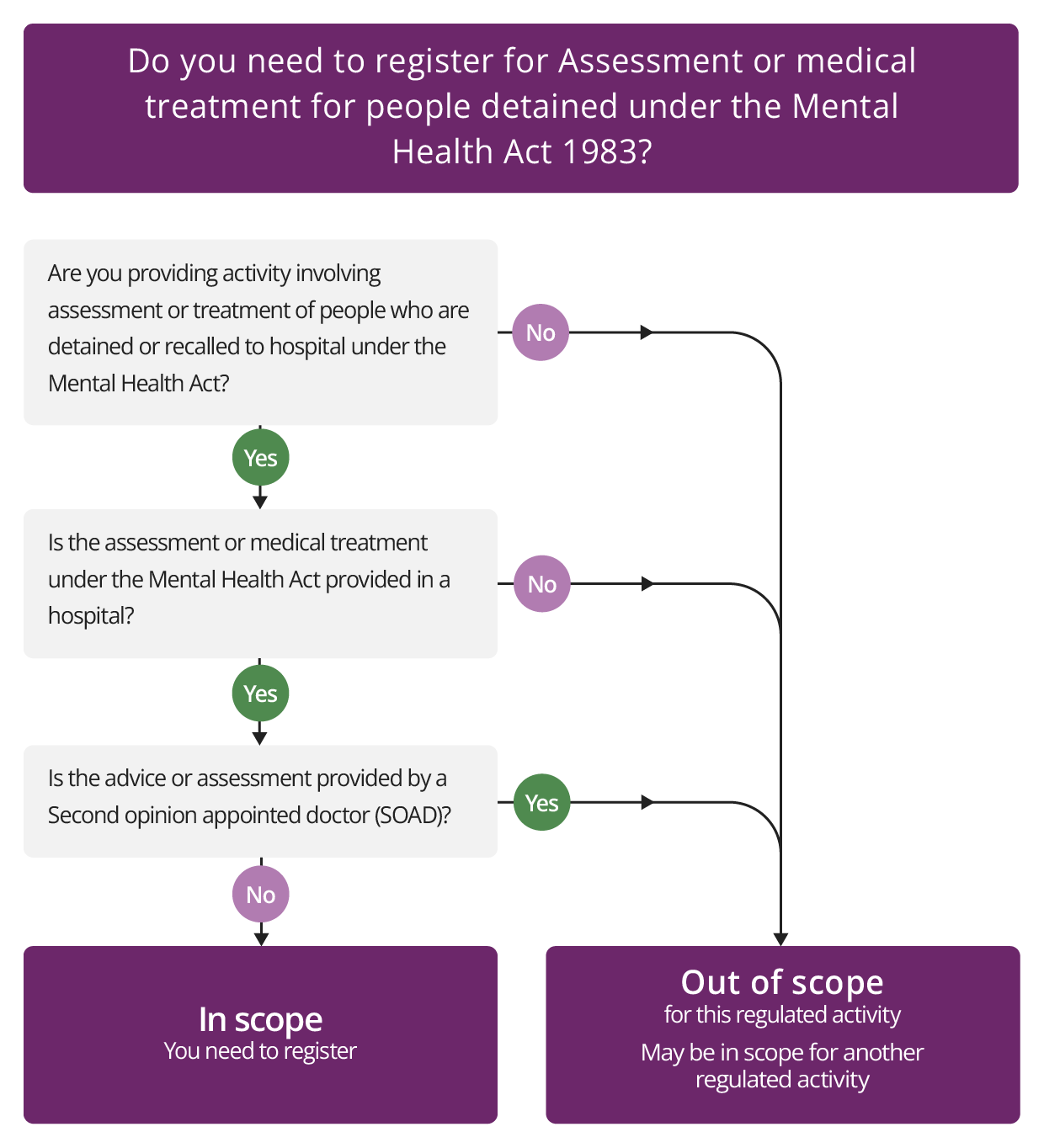
Assessment or medical treatment for people detained under the Mental Health Act 1983
Description
This regulated activity relates to the treatment of people who are detained in, or recalled to, hospital for assessment and/or medical treatment under the Mental Health Act 1983. This includes people whose initial detention was under another enactment, but which has taken effect as a Mental Health Act detention.
The activity only applies to the use of the Mental Health Act in hospitals, rather than in any other setting.
It includes the use of short-term, emergency holding powers under Section 5 of the Mental Health Act.
Importantly, this means it also applies to hospital services that are not specialist mental health inpatient services, such as acute hospitals, where the Mental Health Act could be used to detain patients for short periods under temporary arrangements.
The regulated activity does not apply to:
- locations that are not a hospital (including prison, community or residential treatment settings for substance misuse or community-based mental health services)
- detention under sections 135 or 136 of the Mental Health Act where people are removed to a designated place of safety (usually a hospital but which, under exceptional circumstances, can be a police cell)
- care homes, even if these have residents who are subject to a community treatment order or guardianship under the Mental Health Act or are on leave from detention in hospital under the Act.
- assessment or treatment by a medical practitioner appointed to provide a second opinion. This means that treatment for the purposes of Part 4 of the 1983 Act in giving a certificate under section 57, 58 or 58A of the Act is exempt from registration (we refer to this as treatment or assessment by a Second Opinion Appointed Doctor or SOAD).
Applying for other regulated activities
If you apply for this regulated activity and you also provide treatment for people who are not detained or not liable to be detained under the Mental Health Act, or informal hospital patients, you may also need to apply for the activity of Treatment of disease, disorder or injury.
You do not need to additionally apply to register for the activities of Personal care or Nursing care if you provide these activities in the delivery of this regulated activity. However, you may need to apply for other regulated activities if you are providing them in separate services.
Medical treatment in relation to mental health and this regulated activity
Medical treatment, only for this regulated activity, is defined in the Mental Health Act 1983 as including:
- Nursing, psychological intervention and specialist mental health habilitation, rehabilitation and care offered to alleviate, or prevent a worsening of, a mental disorder or one or more of its symptoms or manifestations.
Check if you need to register for Assessment of medical treatment for people detained under the Mental Health Act 1983
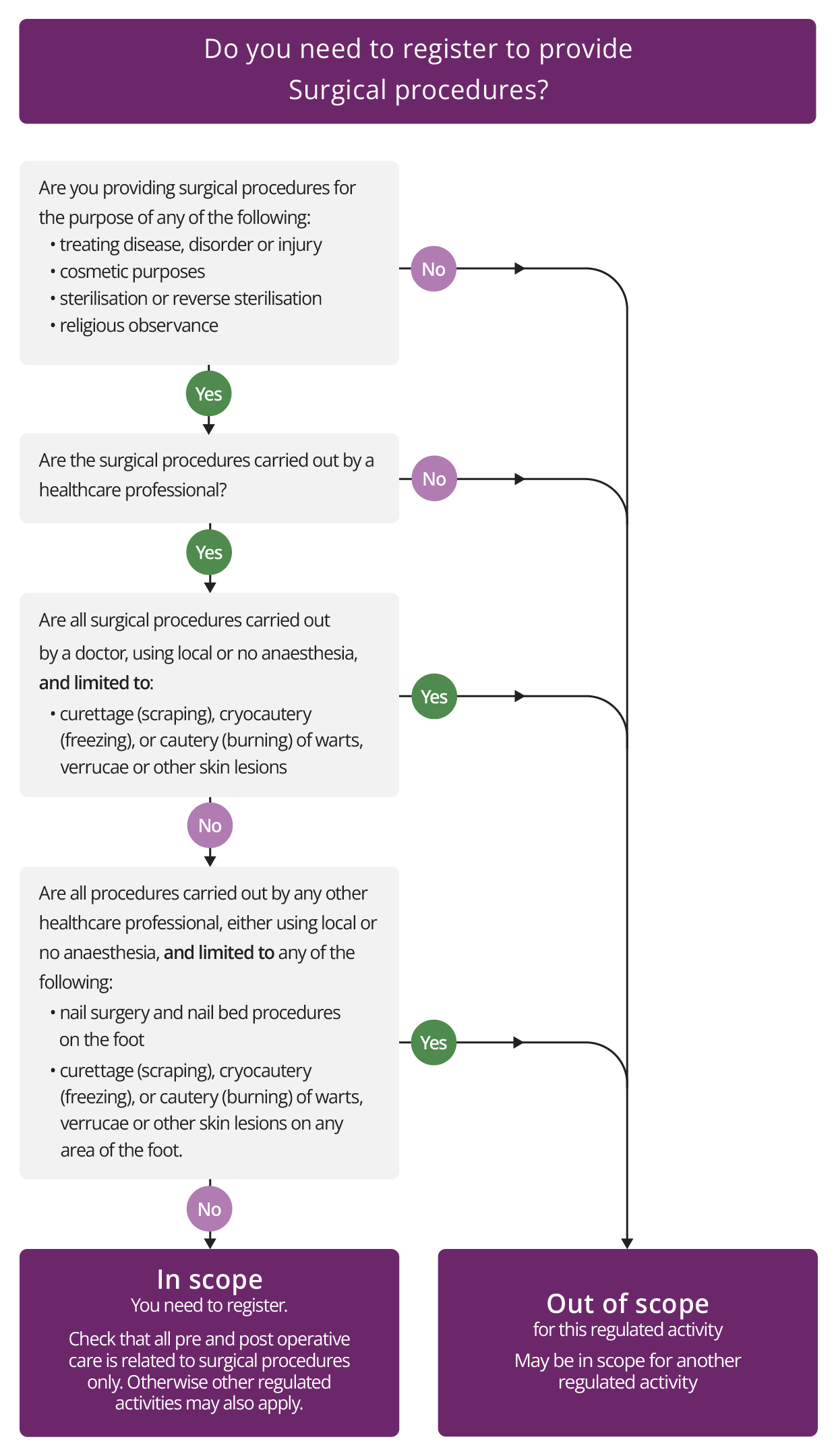
Surgical procedures
Description
This regulated activity covers the following procedures when carried out by a healthcare professional:
- Surgical procedures for the purpose of:
- treating disease, disorder or injury
- cosmetic surgery
- religious observance (for example, circumcision)
- sterilisation or reverse sterilisation.
The activity does not cover the following surgical procedures if they are carried out using local anaesthesia or no anaesthesia:
- nail surgery and nail bed procedures on the foot carried out by any healthcare professional
- curettage (scraping), cautery (burning) or cryocautery (freezing) of warts, verrucae or other skin lesions, carried out by:
- a medical practitioner, or
- another healthcare professional on any area of the foot.
Surgical egg retrieval
This regulated activity does not cover surgical egg retrieval carried out in connection with an activity listed in Schedule 2 of the Human Fertilisation and Embryology Act 1990 for which a licence has been granted under section 16 of that Act. This is because this procedure is carried out to assist a person to become pregnant, rather than to treat a disease, disorder of injury or reverse sterilisation.
Other procedures covered by Surgical procedures
If you provide surgical procedures, you will usually need to register for other regulated activities. For example, if you use imaging techniques during surgery you may need to register for the activity of Diagnostic and screening procedures.
Pre-operative and post-operative care
The regulated activity covers all pre-operative and post-operative care that is associated with the surgical procedures.
An example of pre-operative care might include assessment by an anaesthetist shortly in advance of the procedure (where this is to assess the patient’s suitability directly related to the procedure). It would not include an initial consultation with a surgeon before the procedures had been decided.
For post-operative care, the activity must be related to the procedure to be within the scope of the regulated activity. This will normally mean that it is planned to be related to the procedure. For example:
- post-anaesthetic care (recovery)
- follow-up in an intensive care unit
- care on a ward following surgery
Post-operative care may include a planned follow-up consultation after surgery, but would not include any further additional treatment (apart from checking on the procedures) that is decided in that follow-up consultation.
It also includes other treatment that is directly related to the surgical procedures and carried out under the surgical team. For example, if an anaesthetist temporarily changes a patient’s pre-existing prescription for medicines to avoid any conflict with anaesthetic drugs.
If the treatment goes beyond the surgical team, for example where the patient’s cardiologist changes a prescription – not the anaesthetist – then that is considered to be treatment in its own right rather than associated with the surgical procedures.
The key principles are that the activity must be:
- directly related to the surgical procedures, so only from the point at which the surgical procedures are decided on.
- only the planned pathway of care, not subsequent treatment.
- only within the surgical team, not the activity of other healthcare teams that may be taking place at the same time.
Surgical procedures carried out for religious reasons
Surgical procedures for religious reasons, such as circumcision, are only included where they are carried out by a healthcare professional. Where a healthcare professional carries out surgery for religious purposes they will be acting in their capacity as a healthcare professional rather than in a religious or spiritual role. This is because the code of practice for a registered healthcare professional prohibits them from disregarding the need to have appropriate skills, experience, equipment and facilities for this procedure and they cannot 'opt out' of their core duties and responsibilities as a registered healthcare professional, even if they are acting in a spiritual or religious role.
Cosmetic surgery
The regulations do not define cosmetic surgery, but the procedures that are within the scope of the regulated activity include those described as being:
- carried out by a healthcare professional for cosmetic purposes, where the procedure involves the use of instruments or equipment that are inserted into the body.
As an example, we consider liposuction involving the insertion of instruments into the body to be included in this activity. This is regardless of whether the liposuction is carried out using general or local anaesthesia, or whether the procedure involves the administration of a laser through a cannula inserted into the body.
A procedure such as the external application of ultrasonic energy without any incision or insertion of instruments into the body is not considered a surgical procedure.
The regulated activity of Surgical procedures does not include the following procedures:
- piercing
- tattooing
- subcutaneous injections to enhance appearance
- removal of hair or minor skin blemishes by application of heat using an electric current.
Hospices
A hospice should not normally be registered for the regulated activity of Surgical procedures.
This is because it is unlikely that a hospice would carry out surgical procedures apart from pleural taps and abdominal paracentesis. We consider these as treatment under the regulated activity of Treatment of disease, disorder or injury for the purpose of registration.
Check if you need to register for Surgical procedures
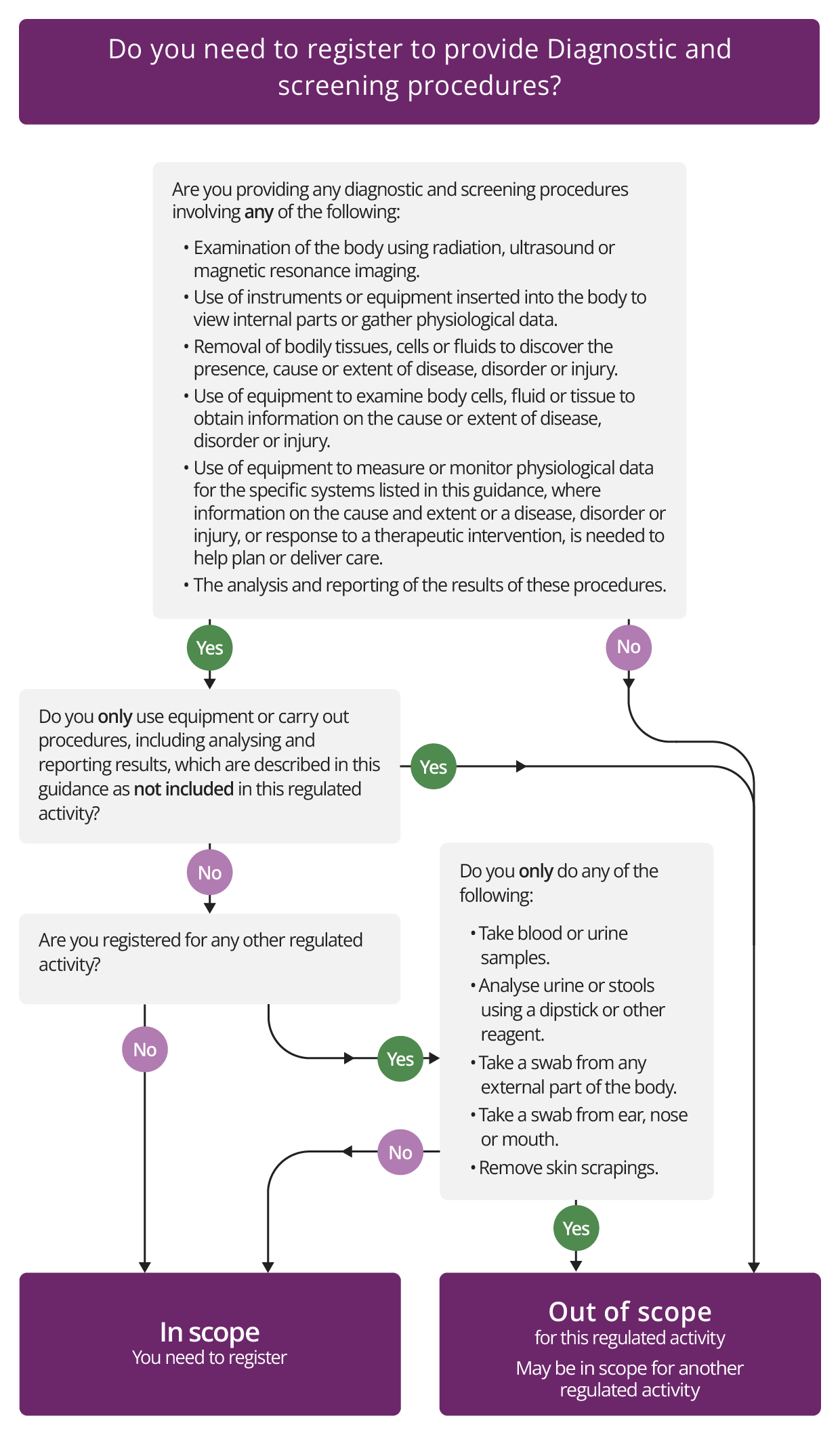
Diagnostic and screening procedures
Description
This regulated activity includes a wide range of procedures related to diagnostics, screening and physiological measurement.
It includes all diagnostic and screening procedures to examine the body that involve the use of any form of:
- radiation (including X-ray)
- ultrasound
- magnetic resonance imaging.
This includes all main forms of diagnostic radiology, radiography and sonography.
But it does not include use of the same technology when it is used for therapeutic purposes, such as radiotherapy or some forms of interventional radiology as these need to be registered for the activity of Treatment of disease, disorder or injury.
The activity of Diagnostic and screening procedures also includes the analysis and reporting of the examinations that are carried out.
If you use the X-ray, ultrasound or magnetic resonance imaging and you also carry out the analysis and reporting, both will be included within a single registration. But if you use a remote contractor for diagnostic analysis, the provider carrying out the analysis and reporting will also need to register in its own right.
Antenatal or baby scans
This activity is not just limited to scans carried out to get a diagnosis – it includes all procedures involving examination of the body by ultrasound, including antenatal ultrasounds scans. For example, an ultrasound performed on a pregnant person for the sole purpose of baby memorabilia or a keepsake (that is, not as part of the maternity pathway) is within the scope of this regulated activity, regardless of whether the scan is being carried out by a qualified sonographer or another person.
Subcontracting arrangements
Where diagnostic images are reported remotely by a subcontracted provider who is outside England, the subcontractor cannot register as they are outside of CQC's remit. However, we will hold to account the registered provider who made the contract with the subcontractor for the way the service is delivered and to make sure there are appropriate arrangements to deliver the service, including arrangements for quality assurance.
What the regulated activity includes
- Most forms of endoscopy. This is included because the activity covers procedures if they involve the use of instruments or equipment that are inserted into the body to:
- view inside of the body, or
- gather physiological data.
- Taking an intraoral scan. This is included because the activity involves the use of equipment inserted into the mouth to create a 3D visualisation of the teeth that can be used to:
- make dental appliances and construct restorations and prostheses
- help to diagnose orthodontic conditions and monitor the progress of treatment.
- Taking a sample or biopsy. This is included because the activity covers procedures if they involve removal of tissue, cells or fluids from the body, for the purpose of diagnosing disease, disorder or injury or monitoring its cause or extent. Therefore, anyone who 'removes' tissue, cells or fluids from the body for diagnostic reasons may need to register.
- Examining a sample. Anyone who uses equipment to examine tissue, cells or fluids from the body to obtain information on the cause and extent of a disease, disorder or injury may also need to register.
If you remove the sample and also carry out the examination, then you can include both in a single registration for the activity. But if you use a remote contractor for diagnostic analysis, such as a laboratory company, that provider will also need to register in its own right. - Physiological measurement (the use of equipment to measure or monitor physiological data). This means obtaining information on the causes and extent of a disease, disorder or injury, or the response to a therapeutic intervention, where the information is needed to plan and deliver care or treatment. It relates to the following systems:
- audio-vestibular
- vision
- neurological
- cardiovascular
- respiratory
- gastro-intestinal
- urinary.
Diagnostic services providing physiological measurement provide a wide range of specialist investigations and procedures that are often an essential part of care and treatment for patients. As well as assessing the function of major organ systems, physiological measurement includes measurement and tests that are part of normal clinical care when carrying on other regulated activities that a provider will already be registered for under the Health and Social Care Act 2008.
What the regulated activity does not include
You will not need to register for this regulated activity if you use:
- an auroscope
- a 12-lead electrocardiograph recording (ECG)
The following physiological tests are not included within the definition of physiological measurement, so you will not need to register if you carry out:
- pulse oximetry when used for 'spot' recording
- peak expiratory flow measured by a peak flow meter
- screening or non-diagnostic spirometry
- non-ambulatory blood pressure recording
- a hearing needs assessment or supply and fit a hearing aid if you are a hearing aid dispenser or are acting under the direction or supervision of a hearing aid dispenser, where:
- the person is aged 19 or over, or
- the person is under 19 and the procedure is carried out in, or arranged by, a school or 16 to 19 Academy.
The following are also excepted from this activity (see the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014, Schedule 1(7)(4)):
- Procedures carried out for research or analysing and reporting such procedures. However, this exception only applies where those research procedures do not form part of a person's care or treatment. As an example, a university with an imaging department that carries out research would need to register with us if it carries out a radiological examination for research purposes that is part of a patient’s care or treatment pathway.
- Taking X-rays by registered chiropractors or the use of ultrasound by registered physiotherapists.
- Carrying out procedures as part of some national cancer screening programmes.
- Fitness screening procedures in a gymnasium, related to the use of fitness equipment or fitness activities, (but treadmill tests for clinical purposes are not exempt).
- Blood tests carried out using a pin prick test or removing blood from a vein where the sample is not sent to a laboratory to be analysed.
- Taking urine samples where the sample is not sent to a laboratory to be analysed.
- Taking and analysing wound swabs.
- Sending samples of body fluids to a laboratory to be analysed, where a provider does not take the samples. For example, when a person produces a urine sample and gives it to a provider, and the provider then sends it away to be tested.
- Procedures carried out by a person in connection with any of the activities authorised by a licence granted by the Human Fertilisation and Embryology Authority.
- Taking or analysing samples of tissue, cells or fluids in order to determine the existence of a genetically inherited disease or disorder, or to determine the influence of a person’s genetic variation on their response to a drug. But these tests are not exempt if carried out as part of:
- planning or delivering the person’s treatment or care, or
- a national screening programme apart from a national cancer screening programme.
You can register for this regulated activity as well as any number of other activities. You do not have to be a healthcare professional to register for this activity.
We will consider certain low-risk procedures as part of a provider's overall registration with us. So, if you are registered with us for any other regulated activity, you will not have to register for the activity of Diagnostic and screening procedures just because you carry out the following procedures:
- taking blood or urine samples
- analysing urine or stools using a dip stick or other reagent
- taking a swab from any external part of the body or from the mouth, ear, nose or throat
- removing skin scrapings.
Organisations providing artificial intelligence software for clinical use
Some organisations that provide artificial intelligence (AI) software will need to register for this regulated activity. It is the use of this technology that may be a regulated activity, rather than the supply of the technology.
A healthcare provider that uses AI technology to deliver a regulated activity will be carrying on the regulated activity and will need to be registered with CQC. However, in some cases, the technology supplier may use its own technology to deliver a regulated activity, independently of any CQC-registered healthcare provider, and will need to register. This is becoming common in diagnostics and screening services, where a healthcare provider sends X-ray, CT or MRI images to an AI supplier that then uses its own AI to analyse the images and report the results. If the healthcare provider does not review the results of the analysis independently, then the AI supplier is likely to be carrying on the regulated activity and will need to register.
Suppliers of AI technology do not need to register with CQC where they only supply their technology to others.
Check if you need to register for Diagnostic and screening procedures
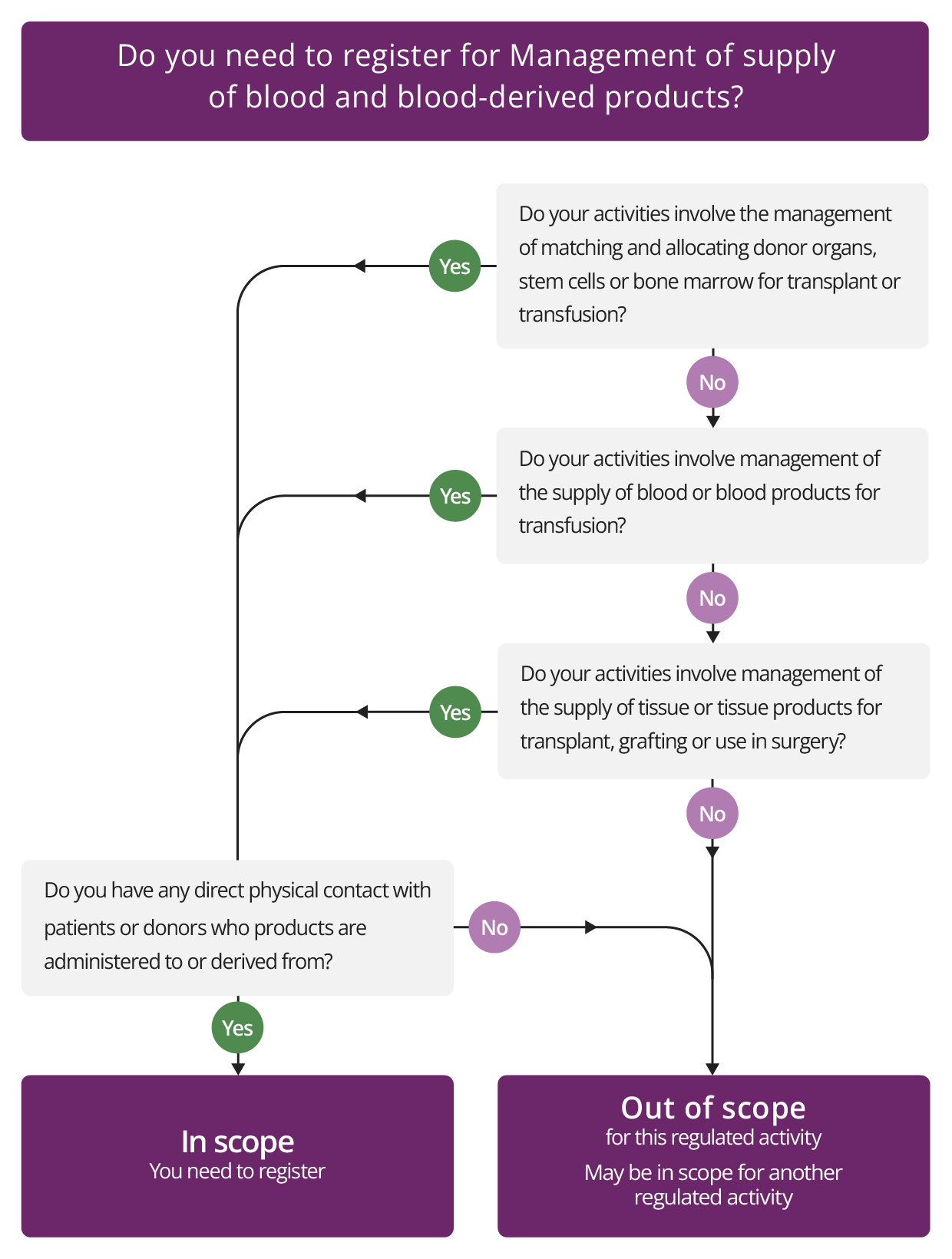
Management of supply of blood and blood-derived products
Description
This regulated activity covers the management of:
- The supply of blood, blood components and blood-derived products for transfusion. Some examples include:
- where NHS Blood and Transplant manages the supply of blood
- a provider managing the supply of blood to another provider
- a service from a dedicated unit, such as a central or regional facility set up to provide this service to individual hospitals in a corporate group.
- The supply of tissues or tissue-derived products for transplant, grafting or use in surgery. For example, this will include supply of organs or tissue by NHS Blood and Transplant or any other provider of transplant organs.
- The matching and allocation of donor organs, stem cells or bone marrow for transplant or transfusion. For example, this will include the role of NHS Blood and Transplant or any other organisation that is involved in managing the supply of donor organs.
What this regulated activity does NOT include:
- The management of supply of blood, blood components and blood-derived products for transfusion, and tissue or tissue-derived products for transplant, grafting or use in surgery where it does not involve direct physical contact with patients or donors.
- How the products are stored, accessed and used in a hospital. This activity is about how blood and tissue products are supplied. Having appropriate equipment and supplies, and storing them, will be part of other regulated activities such as Treatment of disease, disorder or injury or Surgical procedures, rather than an activity in its own right.
- Providing taxi services or other forms of transport that transports blood, organs or tissue products between providers.
- Autologous transplant, where tissue is taken from a person and stored in order to be implanted back into them later. For example, a dental provider removing and storing tissue or bone from a patient and re-implanting it into the same patient at a later date. It also does not include autologous blood transfusion.
- Situations in which a provider’s role is only to remove an organ where the patient has chosen to be a donor. Removing an organ from a donor would be registerable under the regulated activity of Surgical procedures. In this case, a separate agency such as NHS Blood and Transplant will be responsible and would need to be registered for the onward supply of the organ to the transplantation service provider.
- In relation to donor organs, stem cells or bone marrow, the activity covers all of the supply procedures, from donation to matching and allocation, but does not cover the organ demand procedures, such as managing requests or waiting lists for transplantation.
Check if you need to register for Management of supply of blood and blood-derived products
Transport services, triage and medical advice provided remotely
Description
This regulated activity covers two main service types:
- transport (ambulance) services for the primary purpose of carrying a person who requires treatment
- remote medical advice services that give medical advice or triage by telephone or email in cases where immediate action or attention is needed, and are provided by a body established for this purpose.
Transport services
This regulated activity covers services that involve a vehicle designed for the primary purpose of transporting people who need treatment. The nature of the vehicles used determines the need to register.
The term 'designed for' applies to vehicles that are used to transport people who need treatment where this was the manufacturer's original design, as well as vehicles that have been made suitable for this purpose (for example, displaying livery to show what they are and/or being modified).
You need to register for this activity if your transport services are provided in vehicles that meet this definition. This applies whether ambulance transport is the only regulated activity you provide, or if you provide other services as well as transporting patients.
Air ambulances and water ambulances
These are also covered by this regulated activity. But you are exempt and do not need to register for this activity if:
- the aircraft you use is registered with the Civil Aviation Authority and you are not providing treatment to a patient
- the transport is not carried out in England or is carried out under travel insurance arrangements.
Read the General exceptions from registration including Third party exemptions for more information on the exception related to insurance.
What this regulated activity does NOT include
- Transport services provided in vehicles with a different primary purpose (such as taxis, volunteers using their private cars, or mortuary vehicles and Dial-A-Ride vehicles), even though they may be registered with the Driver and Vehicle Licensing Agency as ambulances.
- Search and rescue transport services. This is because the service is provided under arrangements made on people’s behalf by a government department.
Although this regulated activity relates to transport, it does not cover other regulated activities that may be provided in or from a vehicle, such as Treatment of disease, disorder or injury or Diagnostic and screening procedures.
Our view is that this regulated activity will normally cover routine, planned patient transport that is related to treatment.
Sporting or other cultural events
This regulated activity will not apply if you only use a vehicle to transport a person within the boundaries of an event site or venue. As an example, if a person attending or participating in a sporting activity or event needs treatment and is carried in a vehicle from one part of the event ground to another, you do not have to register for that transport. However, if the same situation happens and the person is carried from the event ground to hospital, then you will need to register.
We will take a proportionate and reasonable approach if any emergency, unplanned treatment in this context includes some aspects of other regulated activities on an exceptional basis (such as Diagnostic and screening procedures, Surgical procedures or Maternity and midwifery services). We will also take a proportionate and reasonable approach if, in exceptional circumstances, a provider transports a patient outside an event ground and would not normally consider or plan to do this.
Other regulated activities you may need to register for
Some ambulance service providers may also need to register for the regulated activity of Treatment of disease, disorder or injury. For example, where they employ healthcare professionals and usually carry out treatment.
Where procedures are carried out, such as emergency tracheotomy, insertion of a chest drain or intubation, for registration purposes these would be considered as Treatment of disease, disorder or injury – not as the regulated activity of Surgical procedures.
Where procedures that require specialist surgical training and equipment are an expected part of the service, for example thoracotomy or amputation, we regard these as constituting the regulated activity of Surgical procedures. Therefore, if you expect to carry out such procedures and are equipped to do so you must register for that activity. However, if you carry out emergency procedures unexpectedly, we would take a proportionate view in considering whether this is regulated activity and needs to be registered.
When you register for this activity you will not have to additionally register for the regulated activity of Diagnostics and screening procedures if you only carry out the following diagnostic procedures along with transport:
- electrocardiogram (ECG)
- use of an automated external defibrillator (AED)
- pulse oximetry
- use of a sphygmomanometer
- analysis of urine or stool samples using a dip stick or other reagent
- taking blood, urine samples or swab specimens.
Medical advice provided remotely
This regulated activity applies where medical advice is:
- provided remotely, over the telephone or by email, and
- required in cases that need immediate attention or action, or triage (as opposed to a service where a person submits questions electronically to a provider who responds at a later time, or when a person seeks general health care or lifestyle advice), and
- provided by a body established for that purpose (as opposed to remote consultation and advice by a GP practice or the occasional provision of remote advice by a body such as a hospital or university on an informal basis).
This includes NHS 111 and any other organisation established to provide telephone or internet-based medical advice where immediate action or attention is needed, or that provides triage (see what this means in our glossary of terms).
Ambulance control centres are also covered by this regulated activity where they provide triage using telephony services.
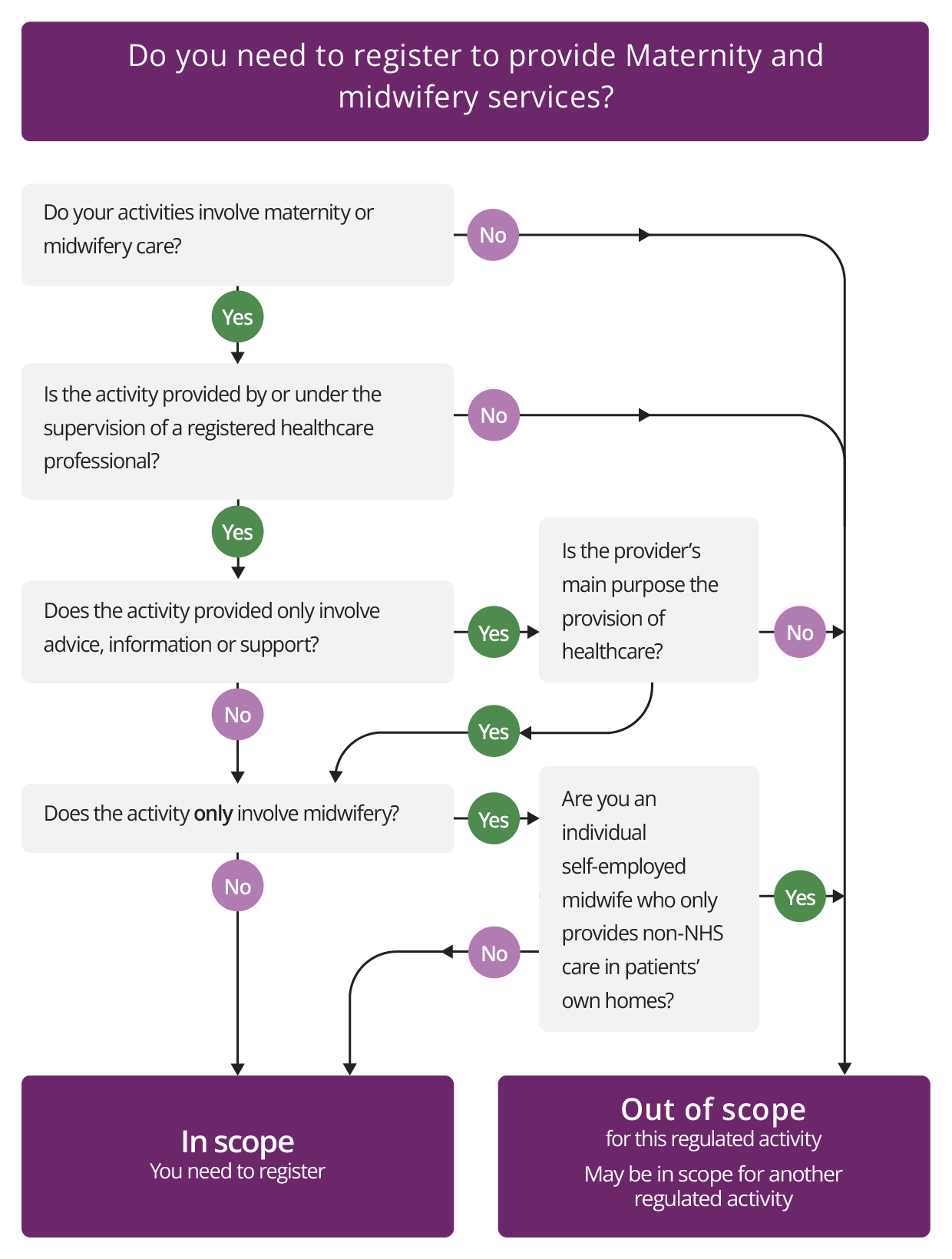
Maternity and midwifery services
Description
This regulated activity covers maternity and midwifery services where they are carried out by, or under the supervision of, a registered healthcare professional.
You do not have to register for this regulated activity if you only provide advice, support or information related to childbirth and parenting, and providing health care is not your main purpose.
An organisation that provides this advice and is not primarily a healthcare provider (such as the National Childbirth Trust) does not need to register, even if the advice is provided by a healthcare professional who it employs.
A hospital provider still needs to register for the activity if it provides advice, because its main purpose is to provide health care.
This regulated activity does not cover arrangements that local authority social services may make under the NHS Act 2006, for the care of pregnant women and women who are breastfeeding.
Midwifery services
Services provided by midwives are exempt and do not need to register for this activity as long as the midwife is:
- acting on their own behalf (self-employed rather than acting for a partnership or organisation), and
- providing non-NHS care (that is, not under contract for an NHS service), and
- providing services to their patients only in the patient’s home and not as part of a hospital or clinic-based service.
This exemption only applies if all these circumstances are met.
Midwives who provide independent maternity and midwifery services exclusively in people’s own homes and who also carry out frenulotomy (tongue-tie treatment) procedures must register to carry on the regulated activity of Surgical procedures.
Antenatal care or postnatal care
Where antenatal or postnatal services are provided as part of primary medical care, the primary medical provider should register for Maternity and midwifery services. Where maternity services are provided as a community or outreach service, the provider will probably need to register for this regulated activity unless they only provide advice and are not primarily a healthcare provider.
The Health Care and Associated Professions (Indemnity Arrangements) Order 2014, is relevant to many healthcare professionals, including individual midwives that are exempt from registering for this activity.
Check if you need to register for Maternity care and midwifery
Termination of pregnancies
Description
This activity includes the termination of pregnancy by surgical or medical methods, including feticide. It does not include advice on termination of pregnancy. The 'morning after pill' is not a form of termination of pregnancy when used as emergency contraception and has its effect before the earliest stages of implantation.
If you are not an NHS body, refer to Regulation 20 of the Care Quality Commission (Registration) Regulations 2009. These regulations place certain obligations on registered providers.
You may need to register for other regulated activities such as Treatment of disease, disorder or injury if, for example, you provide treatment for sexually transmitted infections alongside termination of pregnancy services.
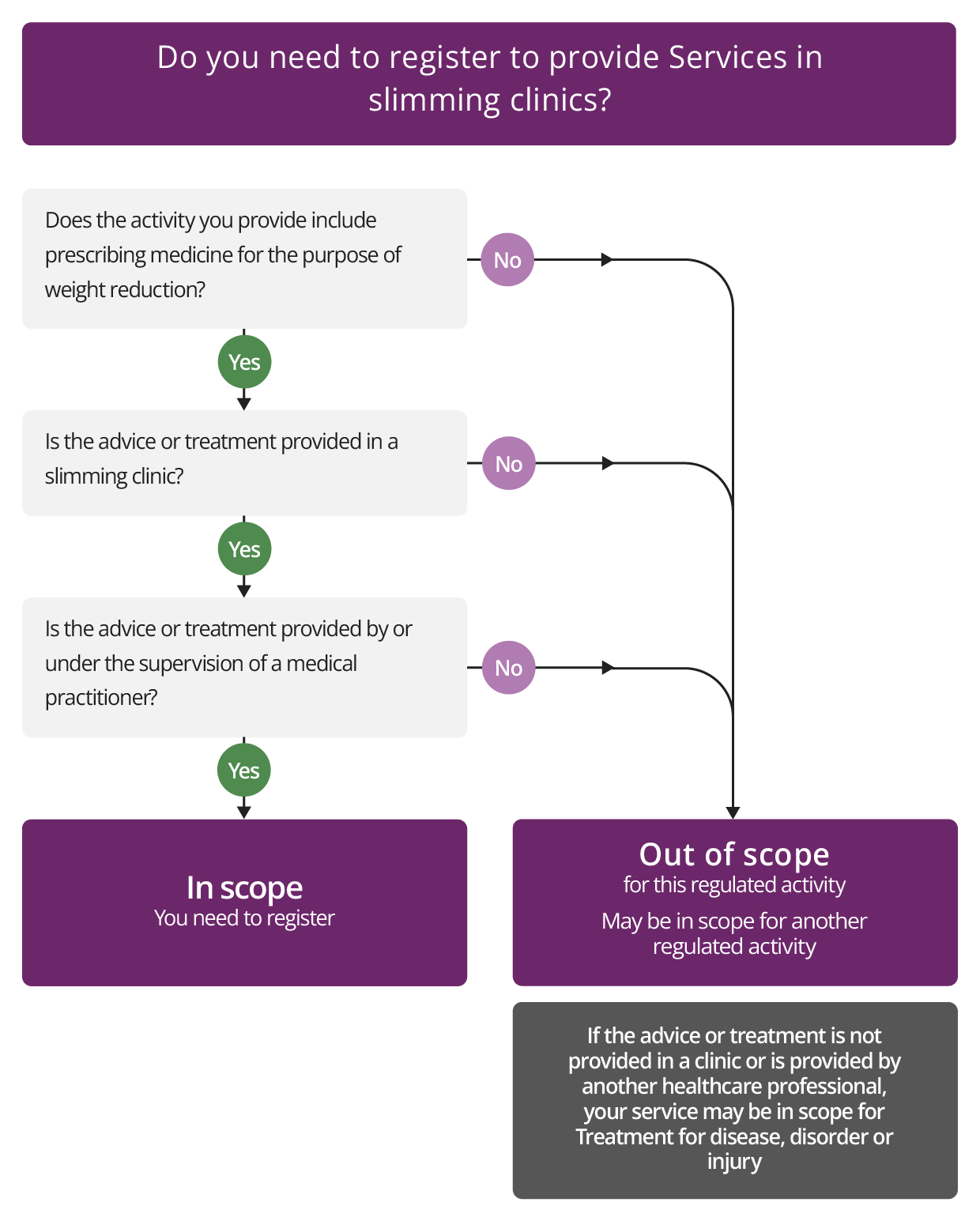
Services in slimming clinics
Description
This regulated activity covers services provided in a slimming clinic that:
- consist of advice or treatment and include prescribing medicines for the purpose of weight reduction, and
- are provided by, or under the supervision of, a registered medical practitioner.
To be registered for this activity, the service must be carried out in a clinic that is a physical location rather than a remote website service.
You may still need to register for other regulated activities. For example, the regulated activity of Treatment of disease, disorder or injury may apply if you also provide treatment for a condition that is not an obesity or weight disorder.
This activity does not apply if a slimming clinic does not prescribe medicines for the purpose of weight loss. For example, a slimming clinic that provides diet plans written or authorised by a medical practitioner will not need to register unless that service or clinic also prescribes medicine for weight loss.
Where a medical practitioner provides treatment of obesity other than in a clinic (for example, through an online service), the regulated activity Treatment of disease, disorder or injury will apply.
Other healthcare professionals who treat people for obesity
If you are another type of healthcare professional and you treat people for obesity this is included in the regulated activity of Treatment of disease, disorder or injury (see section on Treatment of obesity).
Check if you need to register for Services in slimming clinics
Nursing care
Description
This regulated activity covers nursing care where it is not part of another regulated activity.
Regulation 2(1) of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014 describes nursing care as any service that is provided by a registered nurse and involves:
- providing care, or
- planning, supervising or delegating the provision of care.
If you provide nursing care as a necessary part of another regulated activity, for example Treatment of disease, disorder or injury, there is no need to register separately for Nursing care. This regulated activity normally covers services that do not constitute treatment.
Most treatment or care carried out by a registered nurse will involve another regulated activity. Often this will be Treatment of disease disorder or injury, but other regulated activities may also apply. For example, health visiting may include vaccination, which is included in the activity of Treatment of disease disorder or injury, or may include a test that is included in Diagnostics and screening procedures.
What this regulated activity does NOT include
The following types of services are excepted and do not need to register for this regulated activity:
- Nurses' agencies providing agency or locum registered nurses. The supply of registered nurses by an employment agency or employment business to another service provider is not a regulated activity. This exception only applies where the agency is not responsible for directing or supervising the role of the registered nurse in any way.
- Introductory services that connect people with registered nurses. These services provide contact details to enable a person to choose a registered nurse to employ. For example, this might be where a registered nurse is introduced to someone who directs their own care through private funding or a personal budget arrangement. For this exception to apply, the provider of the introductory service must have no ongoing role in the direction or control of the service that the person then receives. Where a person makes a private arrangement and secures a registered nurse for their own care, under their direction, the service provided is exempt even if it did not involve an introductory agency or employment agency. For example, this may include where a person uses a personal budget or a self-pay arrangement.
The above exceptions do not apply to other regulated activities that the registered nurse may be providing. For example, Treatment of disease, disorder or injury.
Check if you need to register for Nursing care

Family planning services
Description
This regulated activity involves services for inserting or removing all types of intrauterine contraceptive system or device by, or under the supervision of, a healthcare professional.
You may also need to register for other regulated activities such as Treatment of disease, disorder or injury if you provide treatment for sexually transmitted infections or menstrual disorders alongside the family planning services.
Next page
Previous page
Download and print
You can download and print a PDF version of the Scope of Registration.